Abstract
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare and life-threatening disease characterized by hemolysis and thrombosis. Many patients with PNH use C5-inhibitors (i.e., eculizumab/ravulizumab) to control their symptoms. Although C5-inhibition prevents intravascular hemolysis (IVH), it fails to prevent extravascular hemolysis (EVH). Because of persistent EVH, up to 72% of eculizumab-treated patients remain anemic, and up to 36% require at least one transfusion per year. Pegcetacoplan (PEG), a C3-inhibitor recently approved by the US FDA to treat adults with PNH, controls IVH and prevents EVH. Studies of PEG treatment in patients with PNH that remained anemic despite eculizumab treatment demonstrated that PEG was superior to eculizumab in achieving improvements in hemoglobin (Hb) levels (Hillmen P, et al., N Engl J Med, 2021 384 (11):1028-1037). Additionally, two early phase open-label trials demonstrated the efficacy of PEG in complement-inhibitor naïve patients with PNH (Wong RS, et al., Blood, 2020 136 [Supplement 1]).
Aims: To present results from the Phase 3 PRINCE study (NCT04085601), a multicenter, randomized, open-label, controlled study evaluating the efficacy and safety of PEG compared to standard of care (SOC; excluding complement-inhibitors) in complement-inhibitor naïve patients with PNH.
Methods: Fifty-three adult (≥18 years old), complement-inhibitor naïve (no complement-inhibitor treatment [i.e., eculizumab/ravulizumab] within 3 months prior to screening) patients with PNH and Hb levels below the lower limits of normal (males: ≤13.6 g/dL; females: ≤12.0 g/dL), and lactate dehydrogenase (LDH) levels ≥1.5 times the upper limit of normal (1.5x ULN; ≥339 U/L) were enrolled. Patients were randomized 2:1 to receive PEG (1080 mg subcutaneously twice weekly [n=35]) or SOC (excluding complement-inhibitors eculizumab/ravulizumab [n=18]) through Week 26. Patients on SOC had the option to switch to the PEG group if their Hb decreased by ≥2 g/dL from baseline. Co-primary endpoints were Hb stabilization (avoidance of a >1 g/dL decrease in Hb levels in the absence of transfusions) and change from baseline (CFB) in LDH level from baseline to Week 26. Secondary endpoints included CFB in Hb levels, transfusion avoidance (defined as the proportion of subjects who did not require a transfusion through Week 26), and the incidence of adverse events (AEs). Statistical analyses were performed using the Cochran-Mantel-Haenszel test and ANCOVA model.
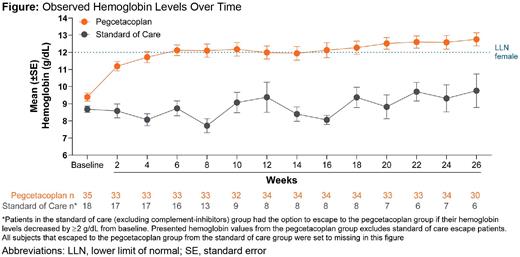
Results: PEG was superior to SOC in both co-primary endpoints. Hb stabilization was achieved by 85.7% (n=30) of PEG-treated patients and 0.0% of SOC patients through Week 26 (p<0.0001). PEG-treated patients demonstrated superior reductions in mean LDH levels from baseline to Week 26 compared to SOC patients (least-squares mean CFB: PEG, -1870.5 U/L; SOC, -400.1 U/L; p<0.0001), and mean LDH levels in PEG-treated patients at Week 26 (mean level: 204.6 U/L) were below the ULN for LDH (226.0 U/L). PEG was also superior to SOC in the secondary endpoints: mean CFB in Hb levels (least-squares mean CFB: PEG, 2.9 g/dL; SOC, 0.3 g/dL; p=0.0019; Week 26 mean Hb: PEG, 12.8 g/dL; SOC, 9.8 g/dL) (Figure) and transfusion avoidance (PEG, 91.4%, n=32; SOC, 5.6%, n=1; p<0.0001). Serious AEs were reported by 8.7% (n=4) of PEG-treated patients and 16.7% (n=3) of SOC patients through Week 26. Two deaths (PEG, 2.9%, n=1, septic shock related to medullary aplasia; SOC, 5.6%, n=1, respiratory failure), both deemed unrelated to treatment, occurred. No events of meningitis or thrombosis were reported in either group. The most common AEs reported during the study were injection site reaction (PEG, 30.4%, n=14; SOC, 0.0%), hypokalemia (PEG, 13.0%, n=6; SOC, 11.1%, n=2), and fever (PEG, 8.7%, n=4; SOC, 0.0%). There were no AEs leading to discontinuation of PEG.
Conclusions: Patients with PNH that were naïve to complement-inhibitor treatment demonstrated meaningful hematological and clinical improvements following 26 weeks of PEG treatment. The safety profile of PEG was similar to previous study results and represent a favorable risk-benefit profile. These results provide evidence for the safety and efficacy of PEG treatment in complement-inhibitor naïve patients with PNH.
Wong: Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis Pharmaceuticals: Research Funding, Speakers Bureau; Alexion: Consultancy, Honoraria, Research Funding, Speakers Bureau. Al-Adhami: Apellis Pharmaceuticals: Current Employment. Ajayi: Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Alvarenga: Apellis Pharmaceuticals: Consultancy. Deschatelets: Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Francois: Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Grossi: Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal